Torishima 5th building, 2-15 Sugawaracho, Osaka 530-0046
Fax: 06-6365-0370
E-mail : info@koeichemicals.co.jp
http://koeichemicals.co.jp
Registered Contract Research Organization approved by Ministry of Health,
Labour and WelfareCa. 1000 human subjects (stratified by age and gender)
registered
Koei Chemical Industry Corporation
Center for Face Survey Cosmetic Skin Medicine
We prove the effects of cosmetics scientifically and clinically employing novel test methodology.
Human monitor tests
Tests for UV-cut effect (standard of Japan Cosmetic Industry Association)
SPF measuring tests
Preliminary test on about 3 subjects
. Main test on 10 subject
Test for the evaluation of effect
However, current status is that there are only a few facilities that can test their products on
Japanese subjects. In our Center for Face Survey Cosmetic Skin Medicine,
research staffs support
our clients to manufacture safe cosmetics for their consumers
PFA measuring tests
Preliminary test on about 3 subjects
Main test on 10 subjects
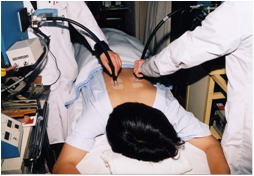
Photograph of a SPF・PFA measuring test
Comparison tests of UV decay (tests for the effect of whitening)
Tests of effect for the use by questionnaires
Other tests for use
Introduction to Contract Research Center of Cosmetics Center
for Face Survey Cosmetic Skin Medicine
This center was established to analyze various data, as a contract research
organization, allergy
and related troubles against developing cosmetics, novel cosmetic products
and basic cosmetics on
skin based upon actual tests on human subjects. The effects of novel cosmetic
products,
on-the-market cosmetics and competitive cosmetics are evaluated by our
company based upon
requests of our clients. Including SPF・PA labeling, patch test-performed
labeling, and allergy test-
performed labeling, a variety of test such as hair tests and tests for
many materials are carried out
by us. In each field of the tests, our expert staffs will do the jobs accurately,
at low price and
confidentially for the needs of our clients so that the results become
their indispensible data.
Patch tests
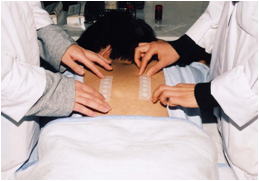
Photograph of a patch test
24-hour patch tests
Cumulative stimulus and sensitization tests (RIPT)
Light patch tests
Cellular and skin model tests other than human subject tests
Safety and effectiveness evaluations are performed based upon up-to-date
skin science
Safety evaluation
successive skin stimulation tests
skin cumulative stimulation tests
skin photosensitization tests
Ocular mucosa stimulation tests
Skin primary stimulation tests
Acute oral toxicity tests
Acute skin toxicity---skin sensitization tests
cosmetic use tests
SPF and PA measurement
Usefulness evaluation
Whitening
Skin wrinkles and furrows
Hair restoration and hair tonic
We present the most suitable tests for usefulness evaluation for the development of new products
Evaluation of anti-oxidation stress agents
Evaluation of anti-aging agents
Evaluation of whitening agents
Evaluation of hair restorers and hair tonics etc.
Information on patch tests
Following the obligatory disclosure of total ingredients of cosmetics,
manufacturers of
cosmetics are apt to be sued by users who claim an allergic reaction, rash
and other consumer
claims. Consequently, corporate themselves have to possess objective evidence
to prove that their
products are safe.